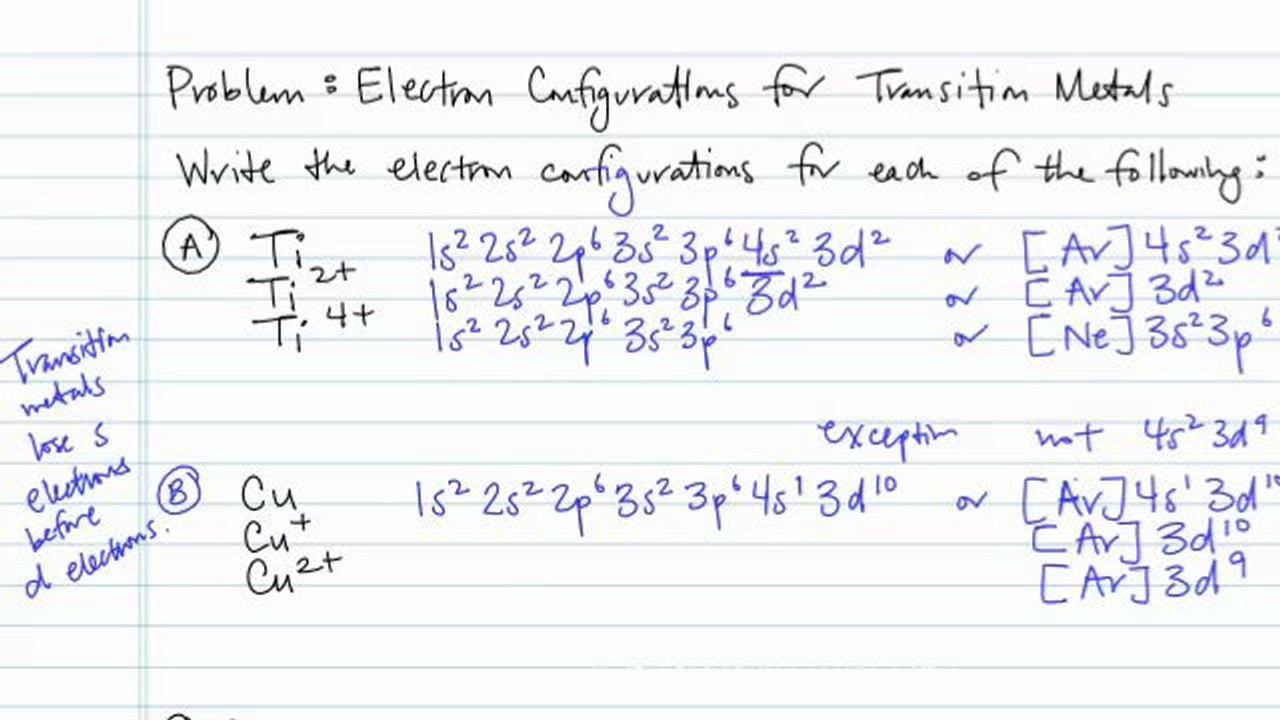
How Many Unpaired Electrons Does Ti2+ Have
The number of electrons in an unpaired state in the last orbital after the electron configuration of an atom is called the valency of that element. In this case the valence electrons of titanium are four.
Electron Configurations For Transition Metals And Their Ions Problem Concept Chemistry Video By Brightstorm
This electron configuration shows that the last shell of the titanium atom has two unpaired electrons.
.png)
. The electron configuration of a neutral titanium atom looks like this. Titaniums atomic symbol is Ti and its atomic number is 22. The highest-numbered shell is the third shell which has 2 electrons in the 3s subshell and 3 electrons in the 3p subshell.
Titanium ion Ti 2 Ti 3 Ti 4 electron configuration. Ti has ___ in its d orbitals. Therefore Ti2 does not have any unpaired electron and v2 have one unpaired electron.
6 What is the electron configuration for Fe 3. How many of the following elements have 1. Which free ion has the greater number of unpaired d electrons Ti2 or Co2.
How many electrons does phosphorus have in its valence shell. Ground state configuration of cobaltAr3d 74s 2Ground state configuration of Co 2Remove 2 electronsSo only one unpaired e Ground configuration of Cr4s 13d 5Cr 34s 13d 2unpaired electrons 3Co 2Cr 313. How many unpaired electrons are the fluorine atom.
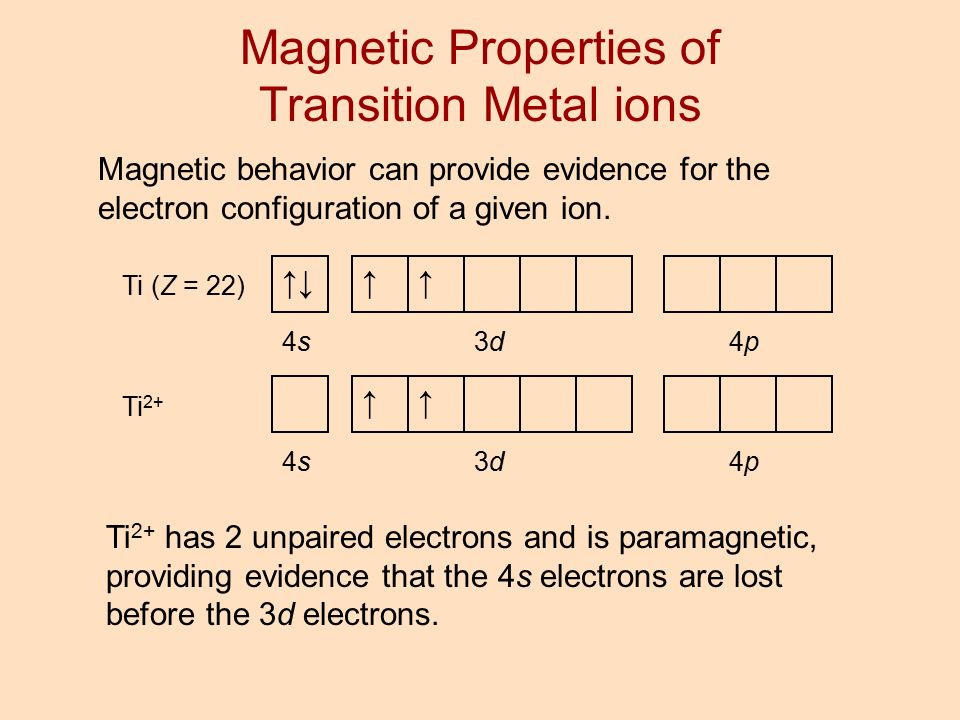
See the answer See the answer done loading. To check for unpaired electrons you only need to check the valence shell or the one that is not filled. V2 will be 1s22s22p63s23p64s03d3 and it will still have three unpaired electrons.
Titanium has three oxidation states. What is the difference between unpaired electrons and lone pair of electrons. It belongs to the transition metal group and can form compounds at an oxidation state of 4.
C Te Hf Si A 1 B 2 C 3 D 4. Which of the following atoms or ions has three unpaired electrons. How many unpaired electrons does an atom of aluminum have in its ground state.
The electron configuration of titanium shows that the last shell of titanium has two electrons and the d-orbital has a total of two electrons. How many unpaired electrons are there in an ion of Ti 2. Ground state electron configuration of titanium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d xy 1 3d yz 1 4s 2.
How many unpaired electrons are in Ti2. They are found in the outermost electron shell of an atom so lone pairs. The atomic number of phosphorus is 15 and the 3p sublevel has three unpaired electrons.
These are 2 3 4. Does F2 have unpaired electrons. Therefore the electron configuration of a neutral titanium atom must account for 22 electrons.
5 How many electrons does an Fe atom have in its 3d subshell number of electrons 3d electrons How many of those electrons are unpaired. A Ar3d2 B Ar4s2 C Ar4s23d2 D Ar4s23d4 E Ar3d4. Titanium has an atomic number of 22.
Math Chemistry Biology Programming Arts History BusinessLanguage Spanish EnglishTipsReviewBlog Home How many unpaired electrons does fluorine have January 21 2022 thanh Please explain these problems1. When transition metals lose electrons they lose the 4s electrons BEFORE the 3d ones so Ti2 will be 1s22s22p63s23p64s03d2 and it will still have two unpaired electrons. In the iron atom there are 4 unpaired electrons.
1s2 2 2s2 4 2p6 10. Valence electrons can be determined by looking at the periodic table. Consequently the electron configuration of the titanium II cation Ti2 must account for 20 electrons since this cation is formed when a neutral titanium atom loses 2 electrons.
Because titanium is four columns from the left it has four valence electrons. How many of the following elements have 2 unpaired electrons in the ground state. Therefore Ti2 does not have any unpaired electron and V2 have one unpaired electron.
V2 have electronic configuration. 7 How many 3d electrons does an Fe3 ion have. Both F2 and F2- will have an odd number of electrons and thus 1 unpaired electron.
Chemistry questions and answers. Choose the ground state electron configuration for Ti2. Choose the ground state electron configuration for Zn2.
Ti2 has 2 unpaired electrons and is paramagnetic providing evidence that the 4s electrons. Ti2 have electronic configuration. Draw the orbital diagram for the d orbitals in an octahedral complex containing.
Solved Question How Many Unpaired Electrons Are There In Chegg Com

Determine Total Number Of Unpaired Electrons In Following Ions Ti 3 V 3 Cr 3 Youtube
.png)
Solved The Ti2 Ion Is Iso Electronic With The Ca Atom A Are There Any Diff Solutioninn
Chapter 8 Electron Configuration And Chemical Periodicity Ppt Video Online Download
No comments for "How Many Unpaired Electrons Does Ti2+ Have"
Post a Comment